Research projects
Human Megakaryopoiesis
Extracellular matrix components
Bone marrow 3D models and biomaterials
Applications
Autocrine regulation and calcium signaling
Platelet Diseases
Myeloproliferative Neoplasms
Drug testing
Research Project
Human megakaryopoiesis
When the lab was founded our goal was to develop protocols for culturing human megakaryocytes
We started with umbilical cord blood which still represents our gold standard and then developed protocols for culturing megakaryocytes from human peripheral blood and bone marrow.
We recently reported (Di Buduo et al, TH 2020) a retrospective analysis of our 15-year experience in processing over 1,500 umbilical cord blood samples, with a focus on experimental procedures that are basic for a reproducible culture of functional megakaryocytes.
Steps of proplatelet formation in human megakaryopoiesis
Research Project
Extracellular matrix components
One of the most abundant components of the bone marrow space, besides cells, is a variety of extracellular matrix components
On selective binding, this environment, in combination with soluble cytokines, regulates haematopoietic progenitor proliferation and differentiation. Haematopoietic progenitors develop as adherent cells in contact with extracellular matrix components in the bone marrow until they are released as non-adherent cells into the circulating blood.
Our lab has pursued for a long time the hypothesis that the interaction of megakaryocytes with bone marrow extracellular matrix components contributes to the regulation of megakaryocyte function. We have demonstrated that some collagen types can support proplatelet formation, while type I collagen is the only extracellular matrix environment that inhibits this process. Our recent evidence indicates that these differences may be ascribed to peculiar structural properties of the collagens, as well as to differences in receptor engagement.
In vivo, we have demonstrated that among bone marrow extracellular matrix components, fibronectin and type IV collagen are the most abundant around bone marrow sinusoids and constitute a peri-cellular matrix surrounding megakaryocytes. Megakaryocytes are able to express components of the basement membrane contributing to their regulation and to bone marrow homeostasis. The production of endogenous extracellular matrix components is significantly boosted in bone marrow regeneration following myelosuppression.
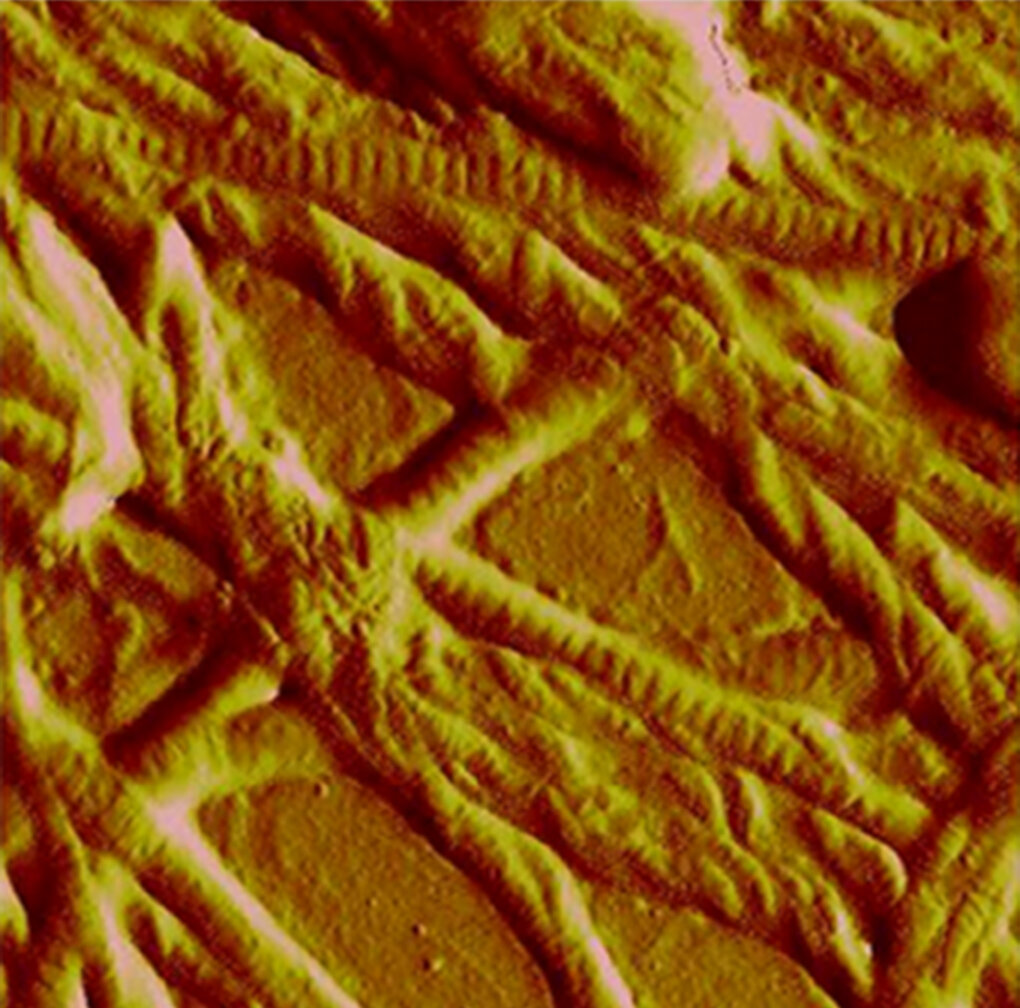
Collagen fibrils imaged by Atomic Force Microscopy
Spreaded megakaryocytes on collagen fibrils
Megakaryocytes express different mechanosensors that contribute to platelet production by regulating the response to extracellular matrix environmental rigidity. Activation of transient receptor potential cation channel subfamily V member 4 (TRPV4), a mechano-sensitive ion channel, is induced upon megakaryocyte adhesion on softer matrices. This response promotes platelet production by triggering a cascade of events that lead to calcium influx, β1 integrin activation and internalization, and Akt phosphorylation, responses not found on stiffer matrices.
By Atomic Force Microscopy we have documented that the tensile strength of fibrils in type I collagen structure is a fundamental requirement to regulate cytoskeleton contractility of human megakaryocytes through activation of the Rho-ROCK pathway and MLC-2 phosphorylation.
Ongoing experiments focus on new mechanosensors, collagens, and fibronectin.
Research Project
Soluble factors and megakaryocyte development
Soluble factors contribute to the regulation of megakaryocyte function in an autocrine manner both in physiology and disease
We have proven that Adenosine diphosphate, released by megakaryocytes, regulates proplatelet formation by interacting with P2Y13. The platelet count of patients with congenital delta-storage pool deficiency, who lack secretable adenosine diphosphate, is significantly lower than that of patients with other platelet function disorders.
Calcium spikes in human megakaryocytes
We have proven the importance of calcium as a fundamental regulator of platelet production. The impact of calcium on megakaryocyte development was also studied in CALR-mutant Myeloproliferative Neoplasms demonstrating that a defective interaction between mutant calreticulin and SOCE proteins promotes megakaryocyte proliferation.
More experiments are ongoing to understand the exact role of calcium in regulating platelet formation in physiology and diseases.
Research Project
Bone marrow 3D models and biomaterials
Millions of platelet transfusions are conducted each year, and the supply of this blood component is limited. There are many diseases where platelet production or function is impaired with severe consequences for patients.
We developed an innovative 3D system to study platelet production that represents the first spatial reconstruction of the bone marrow environment. In this system, human megakaryocytes were able to release functional platelets into vascular tubes.
We use silk fibroin, derived from Bombyx mori silkworm cocoons, as a natural biomaterial to build our models. The useful characteristics of this protein include self-assembly, robust mechanical properties, biocompatibility, and biodegradability. Together with low thrombogenicity, non-toxicity and low-immunogenicity, make this biomaterial a useful blood vessel substitute.
The combination of different bone marrow components and the compliance of silk-based structures makes this model a unique tool for the study of platelet formation and production for use in healthcare needs.
Silk as a biomaterial and silk based 3D bone marrow model.
Megakaryocytes releasing platelets in the 3D silk bone marrow
Now, we are engineering a 3D printed platform that combines the use of silk and 3D printing technology to recreate the environment of the human bone marrow where platelets are produced. We believe that we can provide a sensitive system for ex-vivo screening of new therapeutic options on human megakaryocytes, thus leading to the choice of the best therapeutic course for patients’ clinical care (please go to the drug testing research project).
Applications
Megakaryopoiesis and pathology
Taking advantage of our novel protocol to derive megakaryocytes from peripheral blood, we have recently identified a series of quality and quantitative defects in a patient affected by inherited thrombocytopenias.
We described a defect of proplatelet formation involving tubulin distribution that affects megakaryocytes from patients with heterozygous Bolzano mutation and suggested that this abnormality contributes to their macrothrombocytopenia. We studied proplatelet formation by megakaryocytes carrying the MYH9-RD mutations, which were obtained by culture of patients’ blood progenitor cells. We demonstrated that two different MYH9 mutations cause a myosin-IIA loss-of-function, which results both in a defective regulation of proplatelet formation and in reduced complexity of proplatelet architecture. We propose that these alterations contribute to the thrombocytopenia of MYH9-RD.
Control (left) vs diseased (right) proplatelet forming megakaryocyte
Alterations in megakaryocyte function and proplatelet formation were also demonstrated in von Willebrand Type 2 disease, SLFN14-related thrombocytopenia, ANKRD26-related thrombocytopenia, ETV6-related thrombocytopenia, PTPRJ-related thrombocytopenia, and SRC-related thrombocytopenia.
Applications
Drug testing
Our silk platform represents also a novel screening technology in the pharmaceutical industry to predict the therapeutic effects of compounds on platelet number or function.
We started studying Thrombopoietin mimetic impact on megakaryocyte function showing a different mechanism of action of Romiplostim and Eltrombopag that may be relevant in clinical practice.
We demonstrated that increasing doses of Romiplostim (AMG531) determine a progressive increase of megakaryocyte proliferation with a parallel decrease in megakaryocyte ploidy and the capacity of extending proplatelets. While Eltrombopag favors human megakaryocyte differentiation and platelet production in a dose-dependent manner. These effects are determined by different phosphorylation of AKT and ERK1/2 signaling molecules, which have been proven to be crucial in regulating physiologic thrombopoiesis.
Applications
Myeloproliferative Neoplasms
We are searching the mechanisms of bone marrow fibrosis and megakaryocyte functional alterations in Myeloproliferative Neoplasms
Fibronectin
We identified the role of the fibronectin isoform EDA in regulating hemopoiesis in the bone marrow and demonstrated that this isoform is increased in patients with Primary Myelofibrosis both in the bone marrow and plasma, correlating with the fibrotic phase. We found that mice constitutively expressing the EDA domain (EIIIA+/+), but not EDA knockout mice, are more prone to develop BM fibrosis upon treatment with the thrombopoietin mimetic romiplostim (TPOhigh). Mechanistically, EDA FN binds to TLR4 and sustains progenitor cell proliferation and megakaryopoiesis in a TPO-independent fashion, inducing LPS-like responses, such as NF-κB activation and release of profibrotic IL-6.
Control (left) vs diseased (right) proplatelet forming megakaryocyte
Calcium
Approximately one-fourth of patients with essential thrombocythemia or primary myelofibrosis carry a somatic mutation of the calreticulin gene (CALR), the gene encoding for calreticulin. A 52-bp deletion (type I mutation) and a 5-bp insertion (type II mutation) are the most frequent genetic lesions. We studied the interaction between calreticulin and store-operated calcium (Ca2+) entry (SOCE) machinery in megakaryocytes from healthy individuals and from patients with CALR-mutated myeloproliferative neoplasms. In normal megakaryocytes, calreticulin regulates the activation of SOCE by interacting with ERp57 and STIM1. In CALR-mutated Myeloproliferative Neoplasms, defective interaction between mutant calreticulin and SOCE proteins promotes megakaryocyte proliferation.
Ongoing experiments are focused on calcium metabolism and correlation between plasma EDA fibronectin and clinical features in Primary Myelofibrosis.